
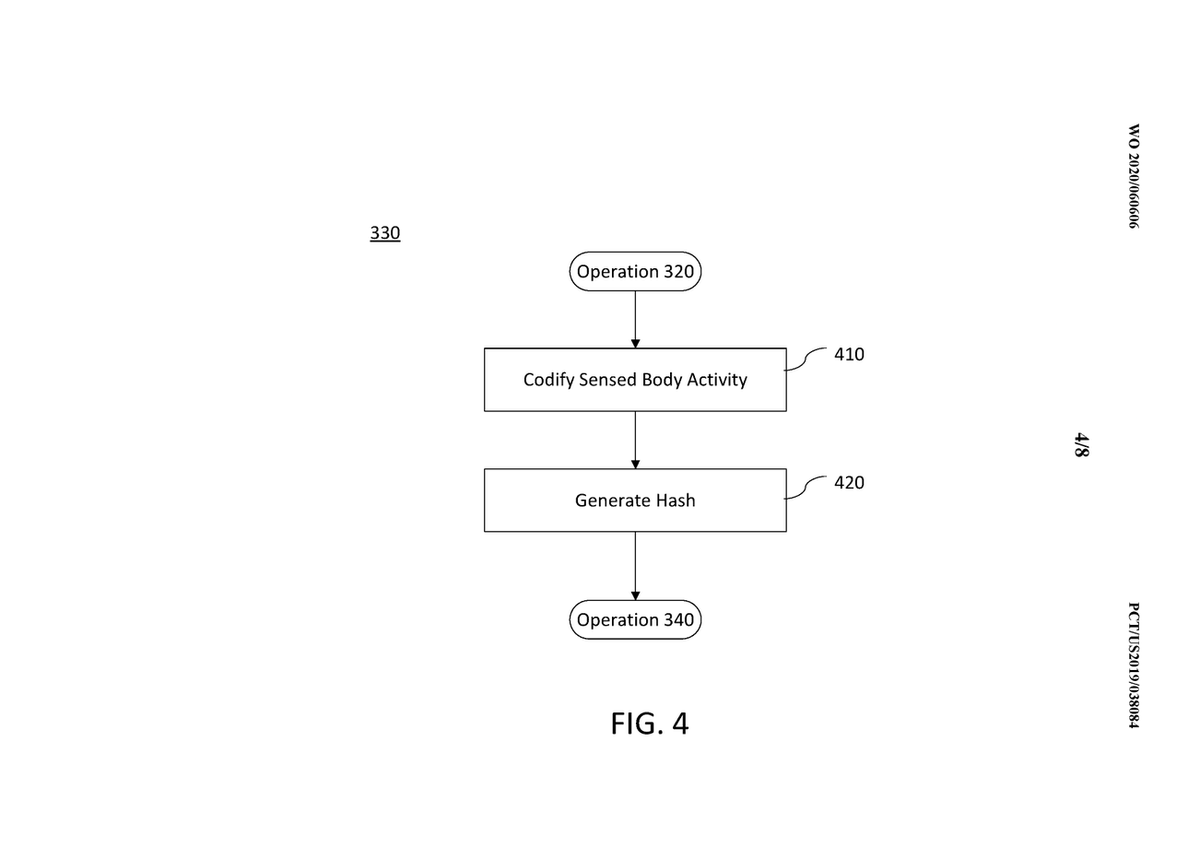
Offers greater flexibility // compared to built-in behaviors. // All the behaviors are defined explicitly. generate ( count: 10, generator: ( i ) => pt. add ( ParticleSystemComponent ( particle: Particle. Only Particles with SingleChildParticle mixin can be used as chainable behaviors. // Expresses the same behavior as above, but with a more fluent API. generate ( count: 10, generator: ( i ) => AcceleratedParticle ( acceleration: randomVector2 (), child: CircleParticle ( paint: Paint (). // Defining a particle effect as a set of nested behaviors from top to bottom, // one within another: // // ParticleSystemComponent // > ComposedParticle // > AcceleratedParticle // > CircleParticle game. Random rnd = Random () Vector2 randomVector2 () => ( Vector2. Any of the approaches could be used in conjunction with existing behaviors where Computed particles in their turn fully delegate implementation of the behavior Chaining allows to express the same composition trees more fluently by defining behaviorsįrom bottom to top. Use behavior chaining (just a syntactic sugar of the first one).Ĭomposition works in a similar fashion to those of Flutter widgets by defining the effect from top Main approaches to implement desired particle effects: Render methods are called during each game loop tick.
DOCS2 PARTICLES UPDATE
When using Particle with a custom Game implementation, please ensure that both the update and ParticleSystemComponent ( particle: CircleParticle (), ), ) add ( // Wrapping a Particle with ParticleSystemComponent // which maps Component lifecycle hooks to Particle ones // and embeds a trigger for removing the component. That's why it is known as dry ice.Import 'package:flame/components.dart' //. This never forms a liquid at atmospheric pressure and always converts directly from solid to vapor. Molecules must be breaking away from the surface as a vapor, because otherwise you wouldn't be able to smell it.Īnother fairly common example (discussed in detail elsewhere on the site) is solid carbon dioxide - "dry ice". For example, naphthalene (used in old-fashioned "moth balls" to deter clothes moths) has quite a strong smell. However, there are some which do easily form vapors. The forces of attraction in many solids are too high to allow much loss of particles from the surface. In most cases, at ordinary temperatures, the saturated vapor pressures of solids range from low to very, very, very low. Sublimation is the direct change from solid to vapor (or vice versa) without going through the liquid stage. Solids can also lose particles from their surface to form a vapor, except that in this case we call the effect sublimation rather than evaporation. As the molecules in the water jostle with each other, new molecules will gain enough energy to escape from the surface. The energy which is lost as the particles evaporate is replaced from the surroundings. Water molecules are simply breaking away from the surface layer.Įventually, the water will all evaporate in this way. If you look at water which is just evaporating in the sun, you don't see any bubbles. That's why, if you look at boiling water, you see bubbles of gas being formed all the way through the liquid.
That's quite different from boiling which happens when there is enough energy to disrupt the attractive forces throughout the liquid. Notice that evaporation only takes place on the surface of the liquid. They evaporate.įig 1: The diagram shows a small region of a liquid near its surface. Some of the more energetic particles on the surface of the liquid can be moving fast enough to escape from the attractive forces holding the liquid together. But within that average, some particles have energies higher than the average, and others have energies lower than the average. The higher the temperature, the higher the average energy. The average energy of the particles in a liquid is governed by the temperature. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behavior of the particles present. The evaporation of a liquid in a closed container.The arrangements of particles in solids, liquids and gases.


 0 kommentar(er)
0 kommentar(er)
